According to the compliance process in the UK, e-cigarette products must be listed in the notification database of the Medicines and Health Products Regulatory Agency (MHRA) of the UK. After the public announcement is completed, it means that these SKUs have passed the compliance review and obtained the legal permission to be sold in the UK market.
To help the industry understand the approval status of new products in the UK market, 2Firsts regularly collates and analyzes the relevant information in MHRA announcements.
The following is the updated notice content from September 15th to September 21st, covering major brands, product types, and preliminary market trend analysis.
During this period, MHRA disclosed a total of 317 registration codes, involving brands including IVG, LOST MARY, FREEMAX, CRYSTAL BAR, VAPES BARS, etc.
New IVG and LOST MARY devices are available
At “Electronic cigarette – Rechargeable, placed on the market with one type of e-liquid (fixed combination). Any rechargeable which can also be used as a refillable should be reported under the refillable category. (That is: rechargeable e-cigarettes sold in fixed combinations with specific e-liquids; If it has the oiling function at the same time, it should be classified into the oiling category. During the period from September 15th to September 21st, a total of 3 registration codes were updated, from the BLACK DRAGON and ORTO brands.
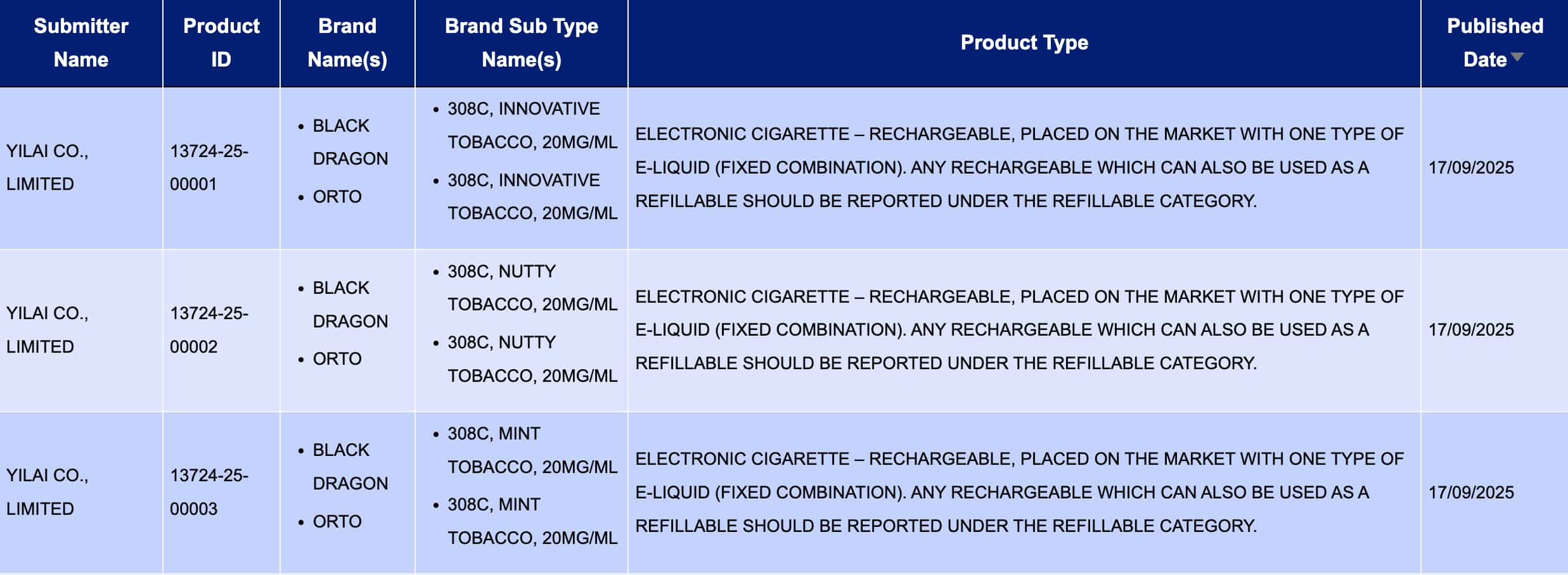
YILAI CO., LIMITED, as the applicant, is making public the 12 registration codes of the BLACK DRAGON and ORTO brands. The products involve three flavors: Innovative Tobacco, Nutty Tobacco, and Mint Tobacco (Innovative tobacco, Nutty tobacco, and Mint Tobacco), with a nicotine concentration of 20MG/ML for all.
At “Electronic cigarette – Rechargeable, device only Any rechargeable which can also be used as a refillable should be reported under therefillable category. Electronic cigarettes – rechargeable, for devices only. Any product that is rechargeable but can also be used as reperfusion should be reported under the reperfusion category. Among the product categories, a total of 4 registration codes were updated during the period from September 15th to September 21st, from brands such as IVG and LOST MARY.
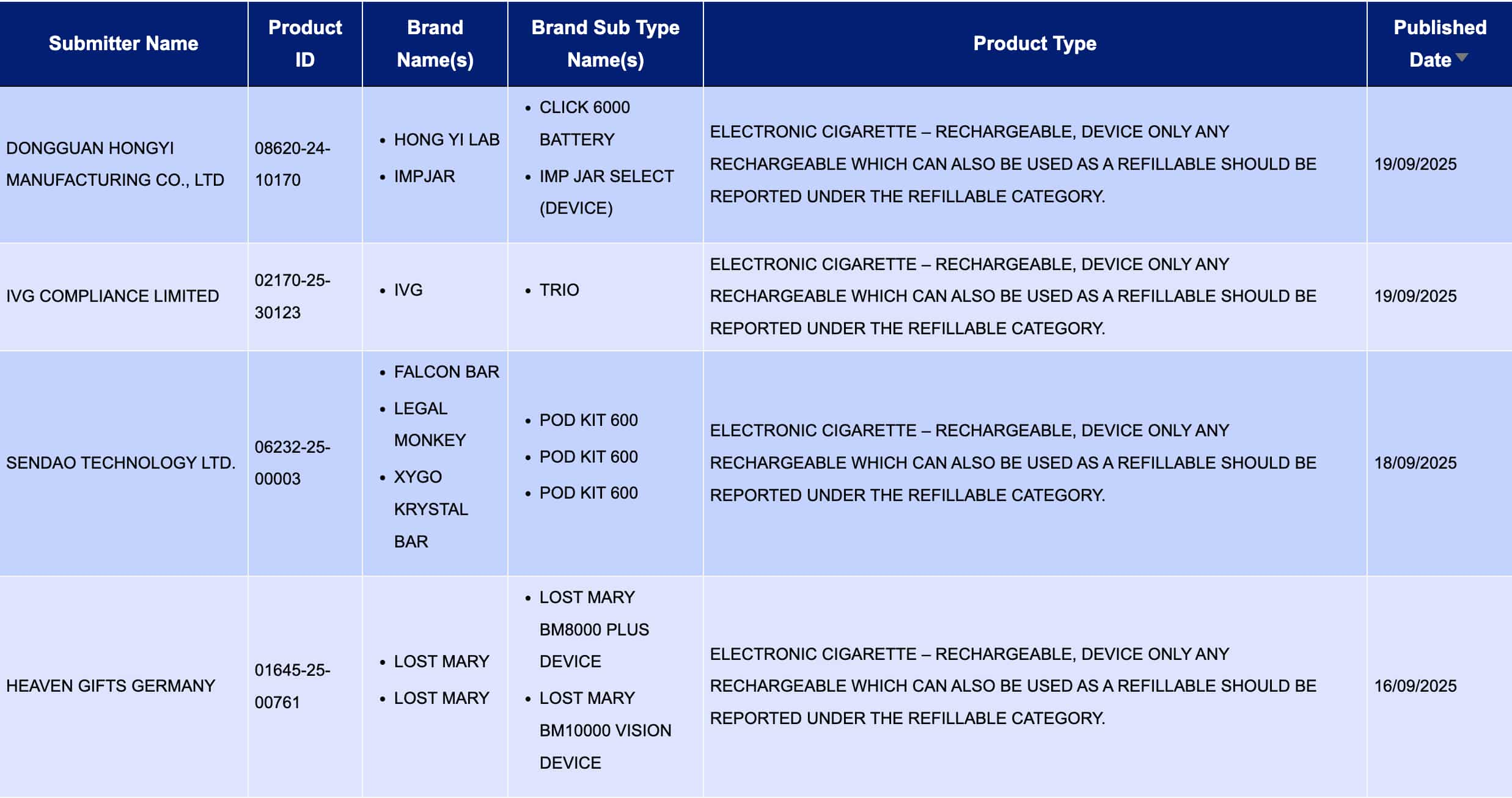
IVG COMPLIANCE LIMITED, as the applicant, brings TRIO of the IVG brand for public notice.
HEAVEN GIFTS GERMANY, as the applicant, is making public the LOST MARY BM8000 PLUS Device and LOST MARY BM10000 VISION Device of the LOST MARY brand.
FREEMAX multi-category centralized public display
In “Electronic cigarette – Refillable, device only.” From September 15th to September 21st, a total of 4 registration codes were updated in the “Reflow E-Cigarette, Device Only” product category, from brands such as FREEMAX and VAPES BARS.
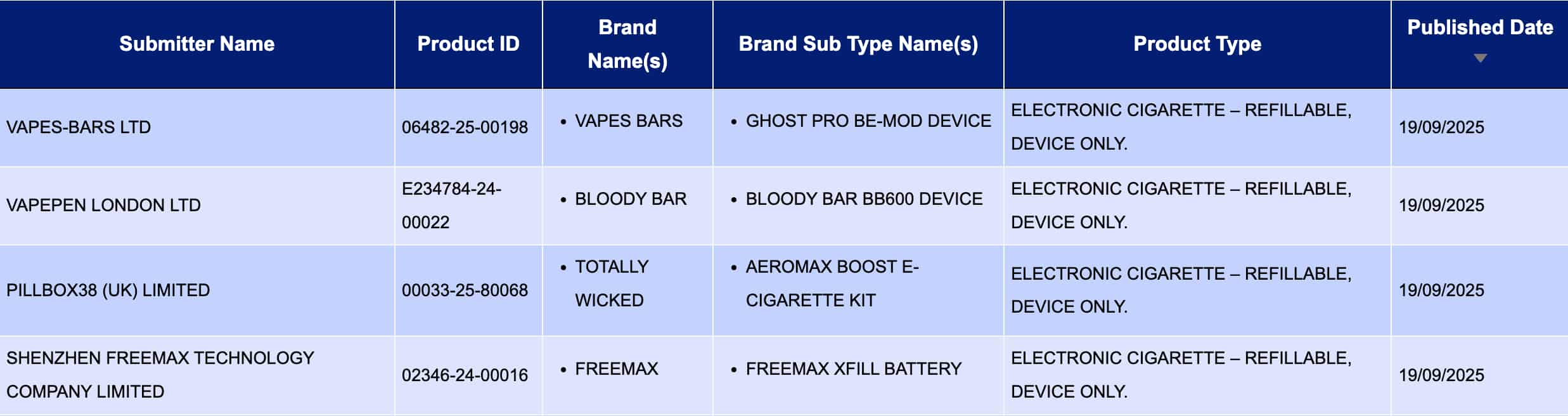
Vapes-bars LTD, as the applicant, is making public the Ghost Pro BE-MOD Device of the VAPES BARS brand.
VAPEPEN LONDON LTD, as the applicant, presents the BLOODY BAR BB600 DEVICE under the brand name of BLOODY BAR.
PILLBOX38 (UK) LIMITED, as the applicant, presents the Aeromax Boost E-Cigarette Kit of Totally Wicked brand.
SHENZHEN FREEMAX TECHNOLOGY COMPANY LIMITED, as the applicant, presents the Freemax Xfill Battery of the Freemax brand for public notice.
In “Kit – Pack containing more than one different e-cigarette device and/or more than one different refill From September 15th to September 21st, a total of 5 registration codes were updated in the product category of “container/cartridge.” (Device kit: refers to a package containing more than one different e-cigarette device and/or more than one different e-liquid container/cartridge), from FREEMAX and IVG brands.
The list of approved e-cigarettes in the UK by FREEMAX: Multiple categories announced, the number of e-cartridges announced drops by over 80% compared to September 15th – September 21st
The Medicines and Health Products Regulatory Agency (MHRA) of the United Kingdom updated 317 e-cigarette registration codes in its database from September 15th to 21st, including new devices of brands such as IVG and LOST MARY, as well as product updates of other categories such as JUUL2 and FREEMAX. It is worth noting that the number of cartridges disclosed during this period decreased significantly by approximately 82% compared to the previous period.
Special Declaration:
This article is only for internal communication within the industry and does not recommend any brands or products.
2. The pictures presented in this article are only for describing the facts and are not intended as advertisements for any products.
3. Minors are prohibited from accessing this article.
Key highlights
The number of public announcements decreased compared with the previous period: A total of 317 electronic cigarette registration codes were approved from September 15th to 21st.
Brand concentrated efforts: New devices of well-known brands such as IVG and LOST MARY are being publicly displayed in a concentrated manner, and products under the FREEMAX brand are being displayed in multiple categories.
The number of public announcements of cartridges has sharply declined: The number of registered codes for cartridges in this issue has dropped by approximately 82% compared to last week. JUUL brand has once again made public announcements of cartridges after January 2011.
The product categories are comprehensive: covering rechargeable devices, oiled electronic cigarettes, complete sets of equipment and independent components.
September 22, 2025 – According to the compliance process in the UK, e-cigarette products must be displayed in the notification database of the Medicines and Health Products Regulatory Agency (MHRA) in the UK. After the public announcement is completed, it means that these SKUs have passed the compliance review and obtained the legal permission to be sold in the UK market.
To help the industry understand the approval status of new products in the UK market, 2Firsts regularly collates and analyzes the relevant information in MHRA announcements.
The following is the updated notice content from September 15th to September 21st, covering major brands, product types, and preliminary market trend analysis.
During this period, MHRA disclosed a total of 317 registration codes, involving brands including IVG, LOST MARY, FREEMAX, CRYSTAL BAR, VAPES BARS, etc.
New IVG and LOST MARY devices are available
At “Electronic cigarette – Rechargeable, placed on the market with one type of e-liquid (fixed combination). Any rechargeable which can also be used as a refillable should be reported under the refillable category. (That is: rechargeable e-cigarettes sold in fixed combinations with specific e-liquids; If it has the oiling function at the same time, it should be classified into the oiling category. During the period from September 15th to September 21st, a total of 3 registration codes were updated, from the BLACK DRAGON and ORTO brands.
The list of approved e-cigarettes in the UK by FREEMAX: Multiple categories announced, the number of e-cartridges announced drops by over 80% compared to September 15th – September 21st
The latest public notice from MHRA (partial) : Image source: MHRA
YILAI CO., LIMITED, as the applicant, is making public the 12 registration codes of the BLACK DRAGON and ORTO brands. The products involve three flavors: Innovative Tobacco, Nutty Tobacco, and Mint Tobacco (Innovative tobacco, Nutty tobacco, and Mint Tobacco), with a nicotine concentration of 20MG/ML for all.
At “Electronic cigarette – Rechargeable, device only Any rechargeable which can also be used as a refillable should be reported under therefillable category. Electronic cigarettes – rechargeable, for devices only. Any product that is rechargeable but can also be used as reperfusion should be reported under the reperfusion category. Among the product categories, a total of 4 registration codes were updated during the period from September 15th to September 21st, from brands such as IVG and LOST MARY.
The list of approved e-cigarettes in the UK by FREEMAX: Multiple categories announced, the number of e-cartridges announced drops by over 80% compared to September 15th – September 21st
The latest public notice from MHRA (partial) : Image source: MHRA
IVG COMPLIANCE LIMITED, as the applicant, brings TRIO of the IVG brand for public notice.
HEAVEN GIFTS GERMANY, as the applicant, is making public the LOST MARY BM8000 PLUS Device and LOST MARY BM10000 VISION Device of the LOST MARY brand.
FREEMAX multi-category centralized public display
In “Electronic cigarette – Refillable, device only.” From September 15th to September 21st, a total of 4 registration codes were updated in the “Reflow E-Cigarette, Device Only” product category, from brands such as FREEMAX and VAPES BARS.
The list of approved e-cigarettes in the UK by FREEMAX: Multiple categories announced, the number of e-cartridges announced drops by over 80% compared to September 15th – September 21st
The latest public notice from MHRA (partial) : Image source: MHRA
Vapes-bars LTD, as the applicant, is making public the Ghost Pro BE-MOD Device of the VAPES BARS brand.
VAPEPEN LONDON LTD, as the applicant, presents the BLOODY BAR BB600 DEVICE under the brand name of BLOODY BAR.
PILLBOX38 (UK) LIMITED, as the applicant, presents the Aeromax Boost E-Cigarette Kit of Totally Wicked brand.
SHENZHEN FREEMAX TECHNOLOGY COMPANY LIMITED, as the applicant, presents the Freemax Xfill Battery of the Freemax brand for public notice.
In “Kit – Pack containing more than one different e-cigarette device and/or more than one different refill From September 15th to September 21st, a total of 5 registration codes were updated in the product category of “container/cartridge.” (Device kit: refers to a package containing more than one different e-cigarette device and/or more than one different e-liquid container/cartridge), from FREEMAX and IVG brands
The list of approved e-cigarettes in the UK by FREEMAX: Multiple categories announced, the number of e-cartridges announced drops by over 80% compared to September 15th – September 21st
The latest public notice from MHRA (partial) : Image source: MHRA
SHENZHEN FREEMAX TECHNOLOGY COMPANY LIMITED, as the applicant, brings the FREEMAX brand:
Freemax EVOX Kit
Freemax REXA S Kit
Freemax Rexa lite Kit
Freemax REXA NANO Kit
A total of 4 registration codes have been updated.
IVG COMPLIANCE LIMITED, as the applicant, presents IVG RELOAD 4 in 1 under the IVG brand.
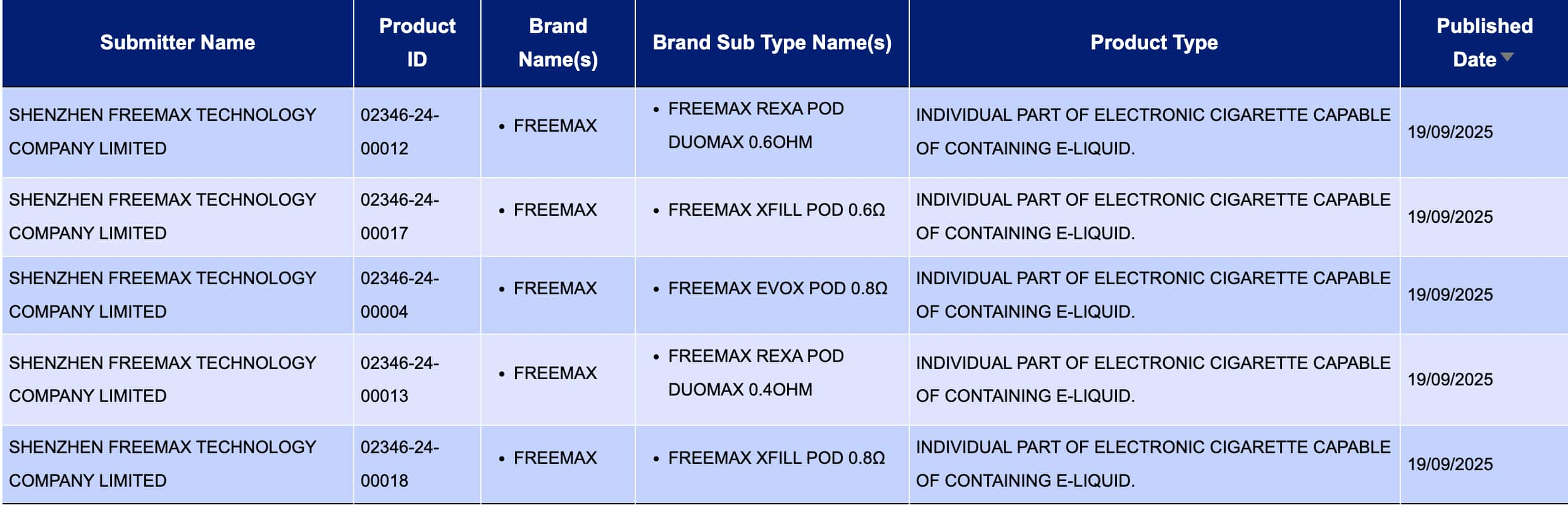
In the product category of “Individual part of electronic cigarette capable of containing e-liquid. (An independent component in an electronic cigarette that can hold e-liquid) From September 15th to September 21st, a total of 46 registration codes were updated, from brands such as FREEMAX and CRYSTAL BAR.

SHENZHEN FREEMAX TECHNOLOGY COMPANY LIMITED, as the applicant, brings the FREEMAX brand:
FREEMAX REXA Pod DUOMAX 0.6ohm
Freemax Xfill Pod 0.6Ω
Freemax EVOX Pod 0.8Ω
FREEMAX REXA Pod D








